Clinical Trials
Launch compliant, cost-effective clinical trials
The Clinical trails help you set up and manage GCP and FDA 21 CFR Part 11-compliant studies faster. Development of the Electronic Data Capture (EDC) system can often take weeks or months, frequently delaying the start of clinical trials and increasing the cost. Designed for research teams instead of programmers, Ledidi Trials help simplify every step so you can focus on the science.
Compliance meets simplicity
Clinical Trials is purpose-built to meet the rigorous requirements for clinical studies in a user-friendly and intuitive interface. The capability ensure your study adheres to the highest regulatory expectations for data integrity, traceability, and security with workflows, user permissions, electronic records, version control, audit trails, and electronic signatures.
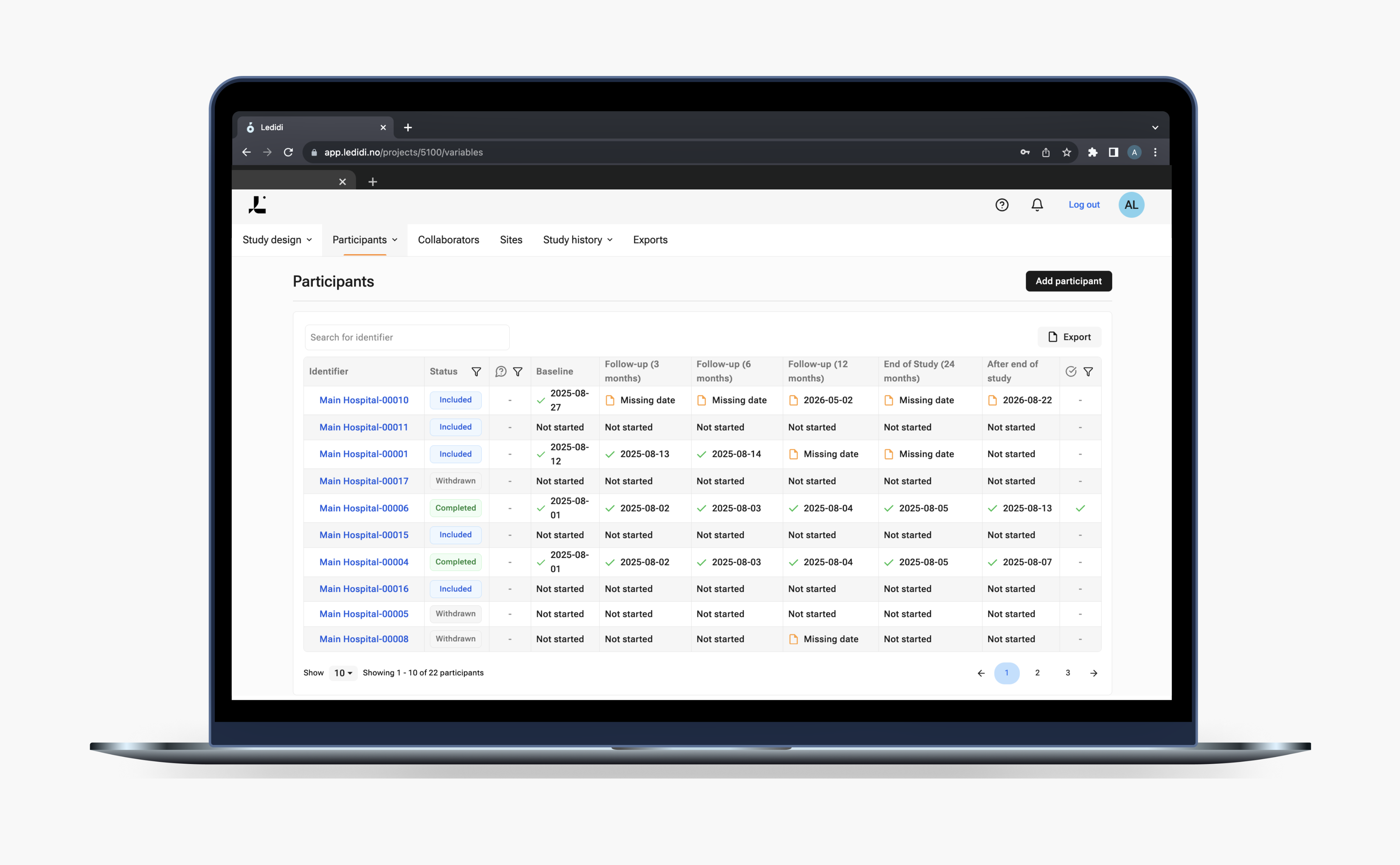
No-code Trial designer
Whether you're running a single-site interventional study or a multi-center clinical trial, the trials capabilities help remove the barriers that typically delay the eCRF setup. No developers. No external vendors. No technical bottlenecks.
With just a few clicks in your browser, you will be able to independently create, configure, and publish your study’s eCRFs according to your study protocol. The capabilities help remove hidden costs, long onboarding cycles, and reliance on third parties. Built for agility, we want to give you full control over your electronic data capture (EDC) solution.
Designed for end users
From Principal Investigators to Study Coordinators, Ledidi trials is built for the people doing the work. The intuitive, clean interface reduces training time and minimises administrative burden across roles.
With usability at its heart, the trials capabilities enable your whole team to focus on research, not on learning a complex system. Everyone—from first-time users to experienced trial professionals—can collaborate confidently, efficiently, and securely.
Audit ready for regulated clinical trials
Ledidi Trials has successfully completed an independent audit confirming its readiness for use in GxP-regulated clinical studies. The assessment verifies compliance with key regulatory expectations, including FDA 21 CFR Part 11, ICH GCP E6(R3), and ISPE GAMP 5.
Key features
-
Randomisation
Ledidi’s integrated randomization functionality enables controlled, auditable participant allocation directly within the eCRF workflow. Built-in block randomisation with site stratification will help maintain balance across treatment arms and study sites
Why it matters: Randomisation is critical to preserving the scientific integrity of a clinical trial by ensuring unbiased allocation of participants across treatment arms.
-
eCRF Setup that mirrors your actual workflow
The trails capabilities is designed to mirror the actual flow of a clinical trial, guiding users through protocol-driven visit structures, participant follow-up, and next-step actions.
Why it matter: This ensures alignment between data capture and real-world study operations.
-
Monitor & Query Management
Real-time, role-specific monitoring tools to review SDV, manage queries, and track completion.
Why it matters: Simplifies oversight, data cleaning, and query resolution in line with GCP quality control expectations.
-
Audit Trail
Track every action — create, read, edit, and delete — with timestamp, user ID, and before/after state. Export complete audit logs to human-readable formats.
Why it matters: Maintains a complete, immutable history of all data operations in the system. Ensures traceability and inspection readiness at all times.








