Medtech & Diagnostics
Evidence generation built for Medtech innovation
Generate regulatory-ready clinical evidence faster — without giving up control of your data.
Medtech evidence comes with unique challenges
Medtech teams operate at the intersection of innovation, regulation, and clinical practice. Generating high-quality evidence often means balancing speed, flexibility, and compliance with limited internal resources.
Do you experience any of the following challenges?
Demonstrating safety and performance for CE, UKCA, and FDA submissions and MDR and IVDR requirements
Running clinical and post-market studies with lean teams
Collaborating across hospitals, clinicians, and CROs
Managing evolving protocols, products, and datasets
Generating long-term real-world evidence to support market access and reimbursement
How Ledidi supports Medtech teams
Ledidi provides a flexible, sponsor-controlled evidence platform designed to support the full Medtech evidence lifecycle — from early clinical studies to post-market surveillance.
-
Own and reuse your clinical evidence
Design and manage studies on your terms, with full visibility and control over data access. Your evidence remains a strategic asset that can be reused across submissions, markets, and future product iterations.
-
Move faster without compromising compliance
Build structured data capture workflows quickly, adapt studies as products evolve, and maintain audit-ready data aligned with regulatory expectations.
Reduce upfront cost and financial risk
Design and validate studies before activation, avoid per-trial setup fees, and minimise expensive changes later by getting evidence workflows right from the start.
-
Collaborate seamlessly with sites and partners
Enable real-time collaboration with hospitals, investigators, and CROs while preserving clear roles, responsibilities, and oversight.
-
Support real-world and post-market evidence
Easily run registries, PMCF, and real-world evidence studies alongside interventional trials, all within a single platform.
-
Scale as your portfolio grows
Start small and expand confidently, without re-platforming as studies, geographies, or regulatory requirements increase.
Trusted by innovative Medtech teams
Ledidi is used by Medtech companies working with leading hospitals and clinical partners to generate high-quality, regulatory-ready evidence efficiently and transparently.
Built to work with your partners
Ledidi is designed to complement existing delivery models.
Medtech companies can work seamlessly with CROs and clinical sites, while maintaining oversight and control of evidence workflows without disrupting operational responsibilities.
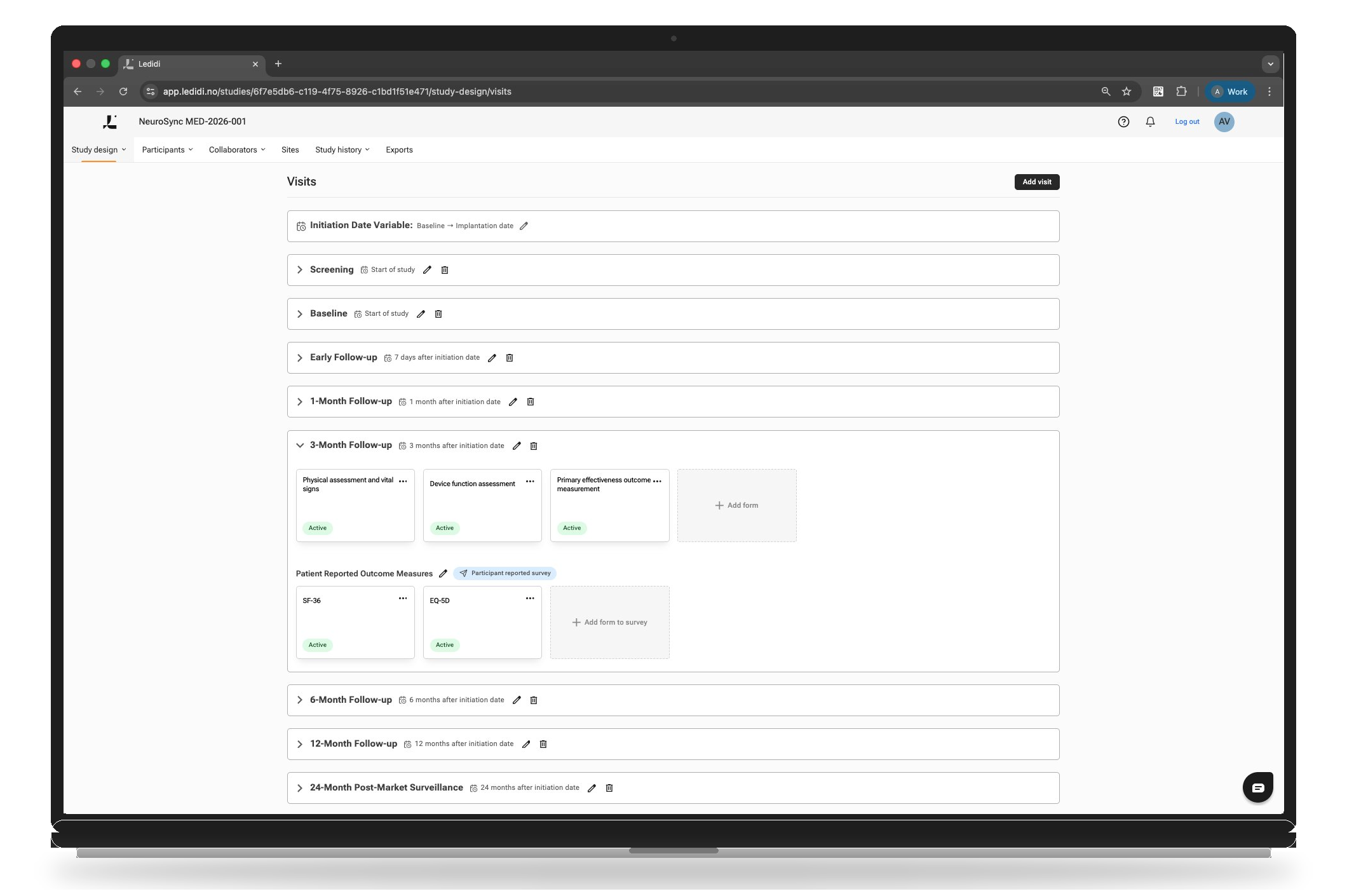
Start building your study before you commit
Design and configure your clinical or post-market study upfront — before incurring trial activation or per-study fees. With Ledidi Core, Medtech teams can validate study design, workflows, and data requirements early, paying only a lightweight platform subscription to get started. This approach reduces upfront risk, avoids costly redesigns later, and gives you confidence that your evidence strategy is fit for purpose before enrolling the first patient.