Capture and analyse research data—without the IT overhead
A cloud workspace built for academic and clinical investigators
Ledidi Core lets research teams design study‑specific forms, collect data under explicit consent, and collaborate across institutions in real time. Leave REDCap set‑ups and spreadsheet work‑arounds behind — focus on science, not systems administration.
Launch studies in days, not months
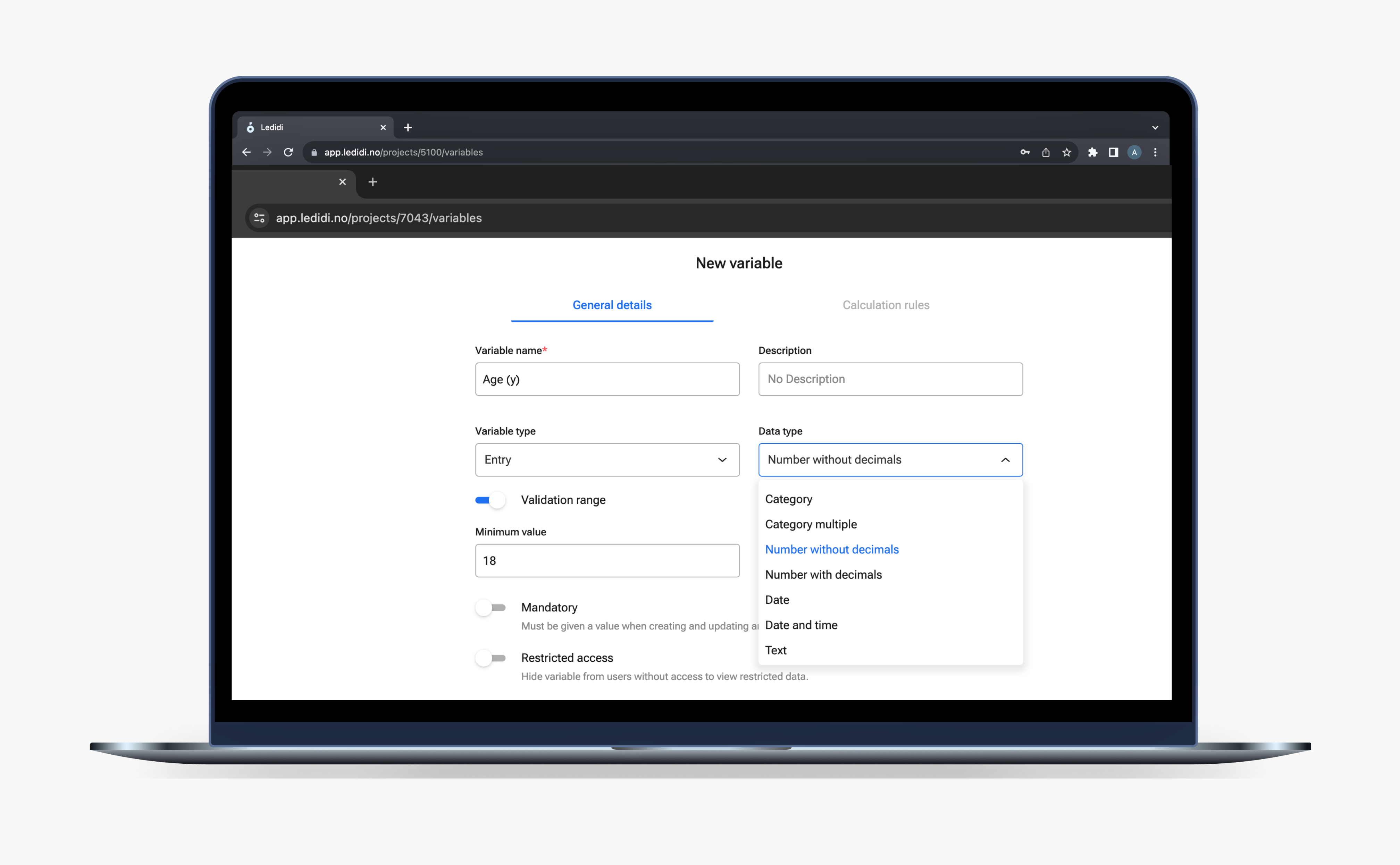
Drag‑and‑drop forms allow you to build protocol eCRFs quickly and reuse templates for future projects.
Collaborate across sites and disciplines
Invite external investigators with role‑based permissions, keep everyone on the same dataset, and track contributions automatically through the audit trail.
Analyse findings inside the platform
Run descriptive and comparative statistics directly on your live database; export tables and figures ready for abstracts and manuscripts—no copy‑paste.
Stay compliant from day one
Explicit‑consent workflows, multi-factor authentication encryption‑in‑use and full audit logs help you meet GDPR and ethics‑committee requirements without extra plug‑ins.
Key features
-
Drag‑and‑drop form builder
Create baseline, follow-up, and outcome CRFs with conditional logic using an intuitive drag-and-drop form builder. Design forms efficiently, set up dependencies between variables, and re-use templates to save time and maintain consistency across your registry projects.
-
Role‑based access control
Apply fine-grained permissions for principal investigators, data managers, and external collaborators through configurable role-based access controls. Define who can view, edit, or share data to ensure privacy and compliance.
-
Built‑in statistics & visualisation
Run descriptive and comparative analyses rendered directly in your project workspace. Ledidi’s statistical analysis tools operate in real-time without exposing sensitive data, producing publication-ready outputs.
-
CSV import & export
Migrate legacy datasets seamlessly via CSV import and export data or visualisations with a single click—ideal for meta-analyses, data sharing, or submissions to national registries.
-
Template management
Re-use study configurations across related projects or multi-arm trials, ensuring methodological consistency and reducing set-up time.
-
Confidential‑computing security
Protect sensitive patient data, even from infrastructure administrators, with confidential computing and encryption-in-use powered by AWS Nitro TEE.
Ready to modernise your research data workflow?


